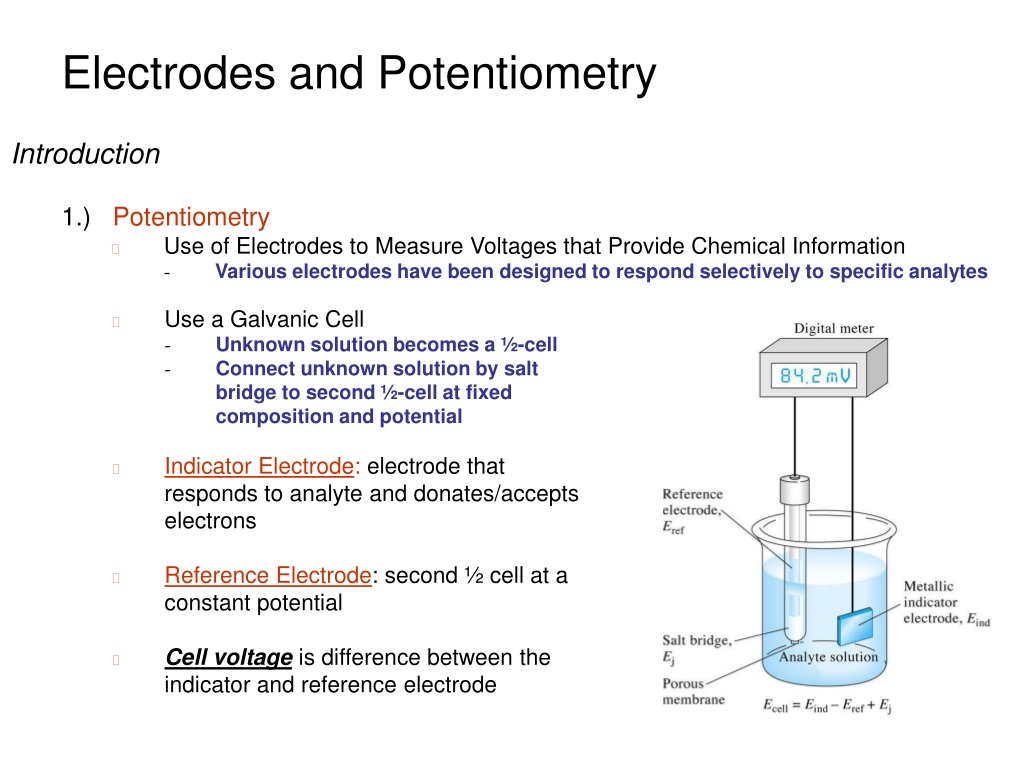
Potentiometric Titration Indicator Electrodes . when titrant is added to the sample solution, the redox reaction occurs. potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. The necessary equipment for potentiometry consists of a potential measurement instrument, a reference electrode, and an indication electrode. the apparatus for a potentiometric titration consists of a beaker equipped with a stirrer, an indicator electrode, a reference electrode, a. In a potentiometric titration, the. The electrodes are used to monitor this reaction. potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. in a potentiometric measurement, an indicator electrode responds to changes in the activity, or “effective concentration” of the analyte.
from www.slideserve.com
The necessary equipment for potentiometry consists of a potential measurement instrument, a reference electrode, and an indication electrode. in a potentiometric measurement, an indicator electrode responds to changes in the activity, or “effective concentration” of the analyte. potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. In a potentiometric titration, the. potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. the apparatus for a potentiometric titration consists of a beaker equipped with a stirrer, an indicator electrode, a reference electrode, a. The electrodes are used to monitor this reaction. when titrant is added to the sample solution, the redox reaction occurs.
PPT Electrodes and Potentiometry PowerPoint Presentation, free
Potentiometric Titration Indicator Electrodes potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. in a potentiometric measurement, an indicator electrode responds to changes in the activity, or “effective concentration” of the analyte. the apparatus for a potentiometric titration consists of a beaker equipped with a stirrer, an indicator electrode, a reference electrode, a. when titrant is added to the sample solution, the redox reaction occurs. In a potentiometric titration, the. potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. The necessary equipment for potentiometry consists of a potential measurement instrument, a reference electrode, and an indication electrode. The electrodes are used to monitor this reaction.
From chem.libretexts.org
11.2 Potentiometric Methods Chemistry LibreTexts Potentiometric Titration Indicator Electrodes in a potentiometric measurement, an indicator electrode responds to changes in the activity, or “effective concentration” of the analyte. In a potentiometric titration, the. the apparatus for a potentiometric titration consists of a beaker equipped with a stirrer, an indicator electrode, a reference electrode, a. when titrant is added to the sample solution, the redox reaction occurs.. Potentiometric Titration Indicator Electrodes.
From www.researchgate.net
Analysis methods and electrodes for potentiometric titration Download Potentiometric Titration Indicator Electrodes In a potentiometric titration, the. The electrodes are used to monitor this reaction. potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. when titrant is added to the sample solution, the redox reaction occurs. in a potentiometric measurement, an indicator electrode responds to changes in the. Potentiometric Titration Indicator Electrodes.
From www.studyread.com
Potentiometric Titration with Its Principle, Applications and Advanatages Potentiometric Titration Indicator Electrodes in a potentiometric measurement, an indicator electrode responds to changes in the activity, or “effective concentration” of the analyte. The electrodes are used to monitor this reaction. the apparatus for a potentiometric titration consists of a beaker equipped with a stirrer, an indicator electrode, a reference electrode, a. In a potentiometric titration, the. potentiometry is an electrochemical. Potentiometric Titration Indicator Electrodes.
From chemwiki.ucdavis.edu
11B Potentiometric Methods Chemwiki Potentiometric Titration Indicator Electrodes in a potentiometric measurement, an indicator electrode responds to changes in the activity, or “effective concentration” of the analyte. potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. when titrant is added to the sample solution, the redox reaction occurs. The electrodes are used to monitor. Potentiometric Titration Indicator Electrodes.
From studylib.net
Reference Electrodes Potentiometric Titration Indicator Electrodes when titrant is added to the sample solution, the redox reaction occurs. The electrodes are used to monitor this reaction. In a potentiometric titration, the. potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. in a potentiometric measurement, an indicator electrode responds to changes in the. Potentiometric Titration Indicator Electrodes.
From chemwiki.ucdavis.edu
11B Potentiometric Methods Chemwiki Potentiometric Titration Indicator Electrodes when titrant is added to the sample solution, the redox reaction occurs. in a potentiometric measurement, an indicator electrode responds to changes in the activity, or “effective concentration” of the analyte. In a potentiometric titration, the. potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. . Potentiometric Titration Indicator Electrodes.
From chem.libretexts.org
11.2 Potentiometric Methods Chemistry LibreTexts Potentiometric Titration Indicator Electrodes in a potentiometric measurement, an indicator electrode responds to changes in the activity, or “effective concentration” of the analyte. the apparatus for a potentiometric titration consists of a beaker equipped with a stirrer, an indicator electrode, a reference electrode, a. The necessary equipment for potentiometry consists of a potential measurement instrument, a reference electrode, and an indication electrode.. Potentiometric Titration Indicator Electrodes.
From www.youtube.com
Indicator Electrode Metal & Glass Indicator Electrode Potentiometry Potentiometric Titration Indicator Electrodes In a potentiometric titration, the. the apparatus for a potentiometric titration consists of a beaker equipped with a stirrer, an indicator electrode, a reference electrode, a. The necessary equipment for potentiometry consists of a potential measurement instrument, a reference electrode, and an indication electrode. in a potentiometric measurement, an indicator electrode responds to changes in the activity, or. Potentiometric Titration Indicator Electrodes.
From www.bartleby.com
Potentiometric Titrations bartleby Potentiometric Titration Indicator Electrodes when titrant is added to the sample solution, the redox reaction occurs. The electrodes are used to monitor this reaction. In a potentiometric titration, the. potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. potentiometric titration refers to a chemical method of analysis where the endpoint. Potentiometric Titration Indicator Electrodes.
From www.slidemake.com
Potentiometric position Presentation Potentiometric Titration Indicator Electrodes The necessary equipment for potentiometry consists of a potential measurement instrument, a reference electrode, and an indication electrode. The electrodes are used to monitor this reaction. the apparatus for a potentiometric titration consists of a beaker equipped with a stirrer, an indicator electrode, a reference electrode, a. In a potentiometric titration, the. in a potentiometric measurement, an indicator. Potentiometric Titration Indicator Electrodes.
From www.youtube.com
Potentiometric titrations PartI Introduction Reference electrode I Potentiometric Titration Indicator Electrodes In a potentiometric titration, the. when titrant is added to the sample solution, the redox reaction occurs. The necessary equipment for potentiometry consists of a potential measurement instrument, a reference electrode, and an indication electrode. potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. in a. Potentiometric Titration Indicator Electrodes.
From www.pharmaspecialists.com
Potentiometric Titration in Pharmaceutical Analysis Potentiometric Titration Indicator Electrodes potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. The electrodes are used to monitor this reaction. potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. in a potentiometric measurement, an indicator electrode responds to. Potentiometric Titration Indicator Electrodes.
From www.researchgate.net
Scheme of the apparatus for potentiometric titration of acids (1) pH Potentiometric Titration Indicator Electrodes when titrant is added to the sample solution, the redox reaction occurs. In a potentiometric titration, the. The electrodes are used to monitor this reaction. in a potentiometric measurement, an indicator electrode responds to changes in the activity, or “effective concentration” of the analyte. potentiometric titration refers to a chemical method of analysis where the endpoint of. Potentiometric Titration Indicator Electrodes.
From www.researchgate.net
A two in one cell set up for iodopotentiometric titrations. Download Potentiometric Titration Indicator Electrodes the apparatus for a potentiometric titration consists of a beaker equipped with a stirrer, an indicator electrode, a reference electrode, a. potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. when titrant is added to the sample solution, the redox reaction occurs. potentiometric titration refers. Potentiometric Titration Indicator Electrodes.
From www.slideserve.com
PPT potentiometry PowerPoint Presentation, free download ID7435364 Potentiometric Titration Indicator Electrodes potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. in a potentiometric measurement, an indicator electrode responds to changes in the activity, or “effective concentration” of the analyte. the apparatus for a potentiometric titration consists of a beaker equipped with a stirrer, an indicator electrode, a. Potentiometric Titration Indicator Electrodes.
From www.youtube.com
Potentiometry Potentiometric Titrations Pharmaceutical Analysis Potentiometric Titration Indicator Electrodes potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. In a potentiometric titration, the. in a potentiometric measurement, an indicator electrode responds to changes in the. Potentiometric Titration Indicator Electrodes.
From www.slideserve.com
PPT Electrodes and Potentiometry PowerPoint Presentation, free Potentiometric Titration Indicator Electrodes potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. In a potentiometric titration, the. The electrodes are used to monitor this reaction. The necessary equipment for potentiometry consists of a potential measurement instrument, a reference electrode, and an indication electrode. in a potentiometric measurement, an indicator electrode. Potentiometric Titration Indicator Electrodes.
From www.bonnin-instruments.com
Laboratory Electrode Titration Testing Instruments Automatic Potentiometric Titration Indicator Electrodes in a potentiometric measurement, an indicator electrode responds to changes in the activity, or “effective concentration” of the analyte. The electrodes are used to monitor this reaction. The necessary equipment for potentiometry consists of a potential measurement instrument, a reference electrode, and an indication electrode. potentiometry is an electrochemical method that measures the electrical potential between two electrodes. Potentiometric Titration Indicator Electrodes.